

The terms “clinical trial” and “study” are used interchangeably in this chapter.Ĭlinical trials are most frequently undertaken in biomedical research, although research that evaluates interventions, usually by comparing two or more approaches, is also conducted in related disciplines such as psychology. Clinical trials may also include questions that are not directly related to therapeutic goals (e.g., drug metabolism) in addition to those that directly evaluate the treatment of participants. Interventions include, but are not restricted to, drugs, radiopharmaceuticals, cells and other biological products, surgical procedures, radiologic procedures, devices, genetic therapies, natural health products (NHPs), process-of-care changes, preventive care, manual therapies, and psychotherapies. As is the case throughout this Policy, the welfare of participants takes precedence over the interests of researchers, institutions, and sponsors.įor the purposes of this Policy, a clinical trial is any investigation involving participants that evaluates the effects of one or more health-related interventions on health outcomes. However, the emphasis in this chapter is on ethics guidance, grounded in the core principles of this Policy: Respect for Persons, Concern for Welfare, and Justice.
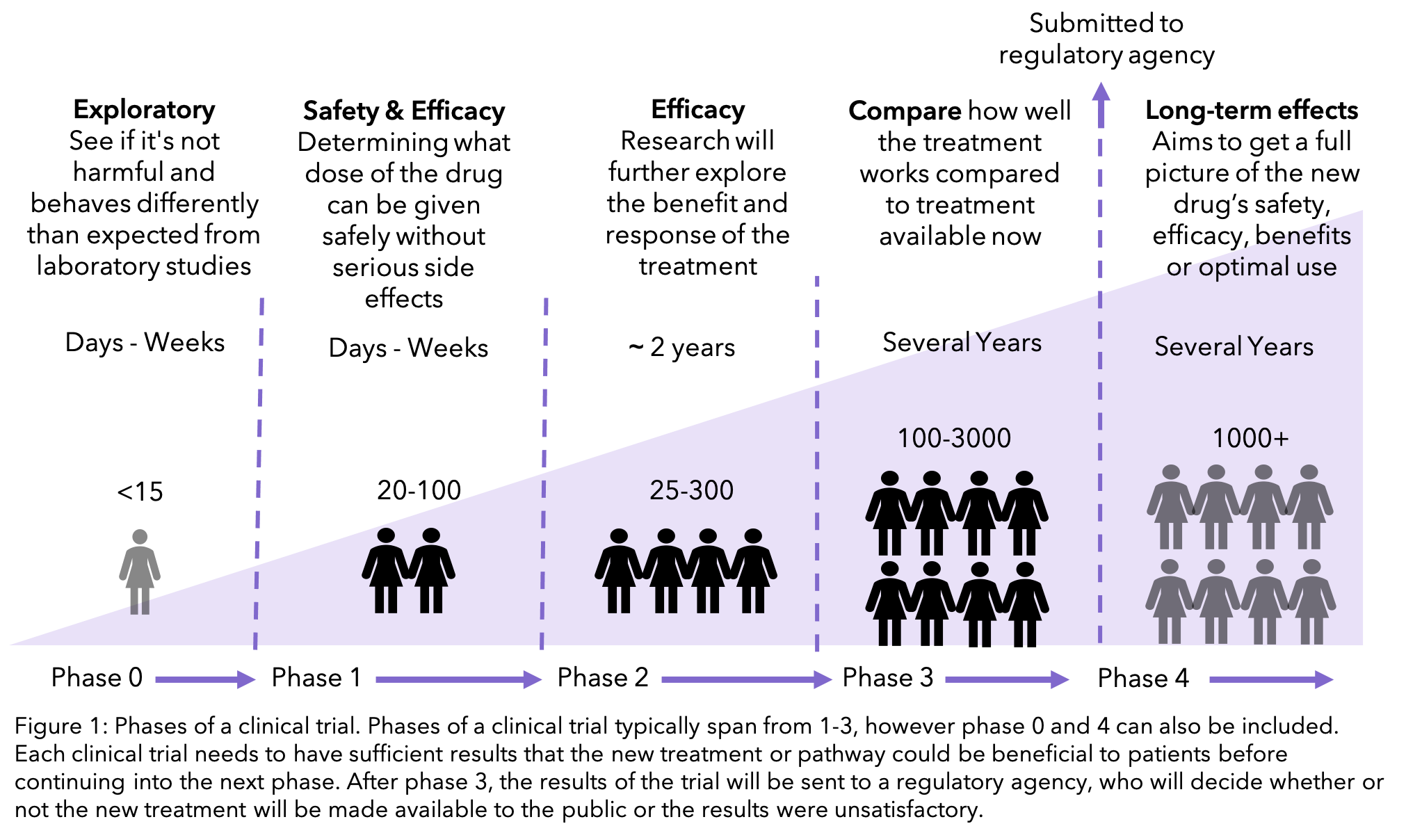
Clinical trials are, perhaps, the most regulated type of research and are subject to provincial, national and international regulatory bodies. In particular, it addresses ethical issues associated with clinical trial design, therapeutic misconception, safety, reporting new information, and registration. This chapter focuses on the ethical issues involved in the design, review and conduct of clinical trials. Safety Monitoring and Reporting New Information
Ethical Issues for Clinical Trial Design and Review TCPS 2 (2018) – Chapter 11: Clinical Trials


 0 kommentar(er)
0 kommentar(er)
